When Taylor Swift dropped Lavender Haze, everyone was in a frenzy and the phrase quickly made its way into playlists and captions everywhere (even in this article). But what does “being in a lavender haze” really mean? And what does any of these have to do with chemistry in the first place?
An expression during the 1950s, “lavender haze” described the dreamy, all-consuming feeling of being in love. But not everybody knows that underneath this romantic metaphor is a scientific truth about lavender which gave way to the expression. The lavender plant, associated with feelings of calm, desire, and emotional warmth, owes much of its reputation to the compounds within it.
And that’s what we’re peeling back today. We’ll explore how the chemistry of lavender connects to the exact feelings Taylor Swift sings about: the feelings of love, longing, and the heady haze of emotion.
Lavender Through Time: Its History & Symbolisms
Lavandula (also known as lavender) was believed to originate from the Mediterranean, Middle East, and India, over 2500 years ago. The Egyptians cherished the lavender plant and used it as a perfume and additive for their mummification process. In fact, archaeologists found traces of lavender when they opened Tutankhamun’s tomb.
The Greeks called lavender “nardus”, derived from the Syrian city Naarda. They considered it as one of the holy herbs used to prepare the Holy Essence. Also, the Greek physician Theophrastus (3rd century BC) wrote about its healing qualities in his book “Concerning Odours”.
The Romans also recognized lavender for its healing and antiseptic qualities, as well as its usefulness as an insect deterrent, and as a detergent. They used lavender to dress the wounds of soldiers and to cook and wash their clothes with it.
During the Middle Ages, many people considered lavender as the herb of love and used it as an aphrodisiac. They also believed that sprinkling lavender water on someone’s head would keep their chastity.
Through time, people continued using lavender for various purposes. Some used it as an insecticide while others utilized its properties as an ingredient for smelling salts. In China, lavender was used in a cure-all medicinal oil called White Flower Oil. Other use cases include embalming corpses, curing animals of lice, taming lions and tigers, repelling mosquitoes, and many more.
Today, lavender has become a symbol of love, devotion, and loyalty. Its association with purity and calmness made it a popular choice as a flower for weddings and engagements. The color of the flower also became a symbol of femininity and homosexuality. In fact, the New York Botanical Garden celebrated lavender as the color symbolic of LGBTQ++ resistance and empowerment.
The Phytochemical Profile of Lavender
The efficacy of lavender comes from its complex blend of volatile organic compounds. Research has shown that major components of lavender oils include:
- Linalyl acetate (30-55%)
- Linalool (20-35%)
- Tannins (5-10%)
- Caryophyllene (8%)
Depending on the species, some may contain trace amounts of sesquiterpenoids, perillyl alcohols, esters, oxides, ketones, cineole, camphor, beta-ocimene, limonene, caproic acid, and caryophyllene oxide.
Linalool
Among the major phytochemicals, linalool gives lavender its signature floral, mildly spicy aroma, and contributes to its anti-microbial activity.
A monoterpene acyclic tertiary alcohol with a global production market valued at $9980 million in 2019, linalool is extremely popular due to its broad spectrum of biological properties – antioxidant, anti-inflammatory, anticancer, cardioprotective, and antimicrobial (Mączka et al., 2022). It is most effective against Gram-negative bacteria. Although its mechanism of action against pathogenic bacteria is still being investigated, one of the key aspects of linalool research is biotransformation.
Lavender plants biosynthesize linalool in both floral and non-floral tissues using isopentenyl pyrophosphate (IPP) and its isomer, dimethylallyl diphosphate (DMAPP), as building blocks (Ana Clara Aprotosoaie, 2014). GPP synthase catalyzes their condensation to form geranyl diphosphate (GPP), the universal precursor of monoterpenes.
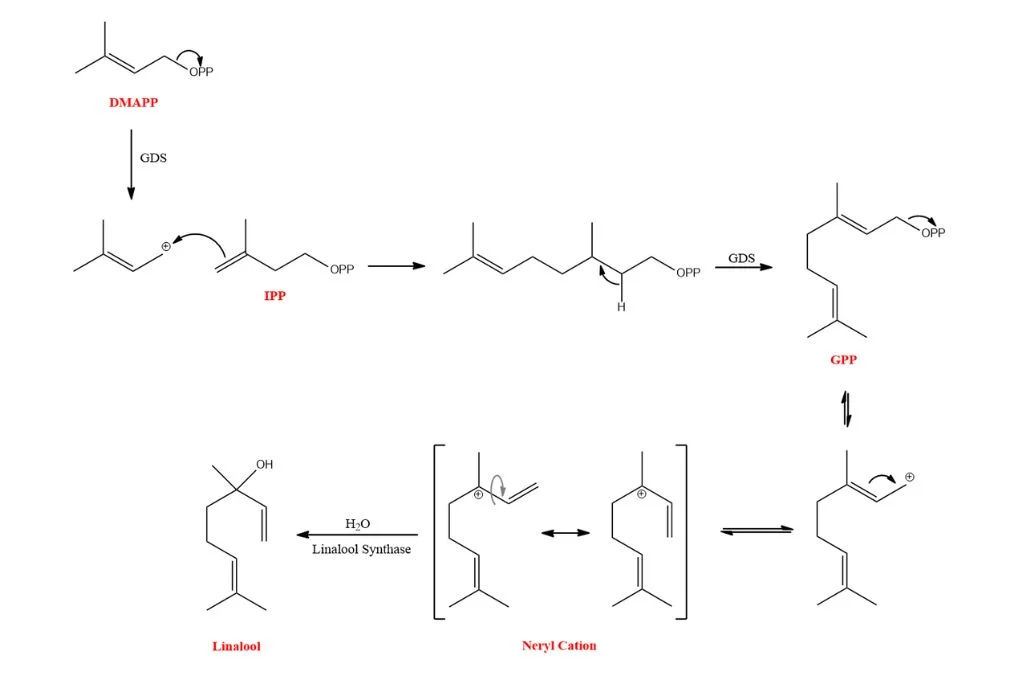
Linalool can also come from other minor biosynthetic pathways as by-products of other processes, such as geraniol and nerol biosynthesis.
Linalool is one of the most studied odorant molecules, not just because of its biosynthetic pathway but also because of its several biological activities. These include the following:
- Sedative Activity: Linalool has been shown to make people feel sleepy and calm. Studies also showed that the more you consume this compound, the stronger its effects will be. A study by Buchbauer et al. (1993) revealed that linalool can help lower body temperatures, increase how long people sleep, and reduce how much they move around.
- Anxiolytic Activity: Lavender oil, which contains linalool, has been used in traditional medicine to help with anxiety. Various studies also support this. For example, lavender oil reduced signs of fear and nervousness in different lab tests designed to mimic anxiety (Umezu et al., 2006; Shaw et al., 2007; Bradley et al., 2006).
- Anticonvulsant Activity: Linalool may also help with seizures. In lab tests with animals, it helped delay the onset of seizures, reduced its duration, and diminished its intensity, showing potential as a natural support for managing epilepsy (Aprotosoaie, 2014).
- Analgesic Activity: Beyond just numbing, linalool also showed potential in reducing the sensation of pain overall. It calms nerves and lowers stress, both of which make pain feel less intense. It also showed promise for helping with nerve pain and sensitivity (Aprotosoaie, 2014).
- Other Benefits: Linalool also showed potential in early studies to fight microorganisms, lower cholesterol, protect cells from damage, and even slow the growth of some tumors.
Linalyl Acetate
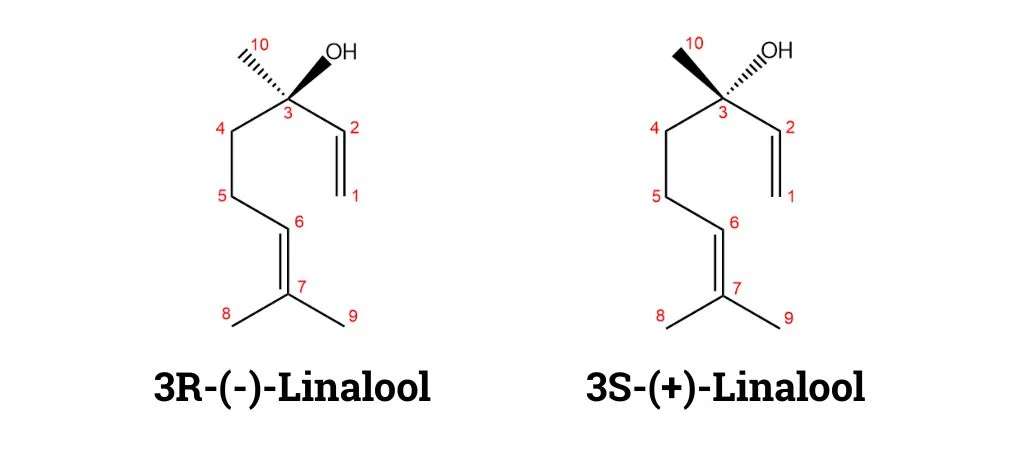
Lavender also contains linalyl acetate which imparts a fruity, sweet fragrance and works in synergy with linalool. It is the monoterpenoid acetate ester of linalool and a phytochemical found in many flowers and spice plants. Like linalool, it also exists in nature as a racemate, comprising equimolar amounts of (R)- and (S)-linalyl acetate.
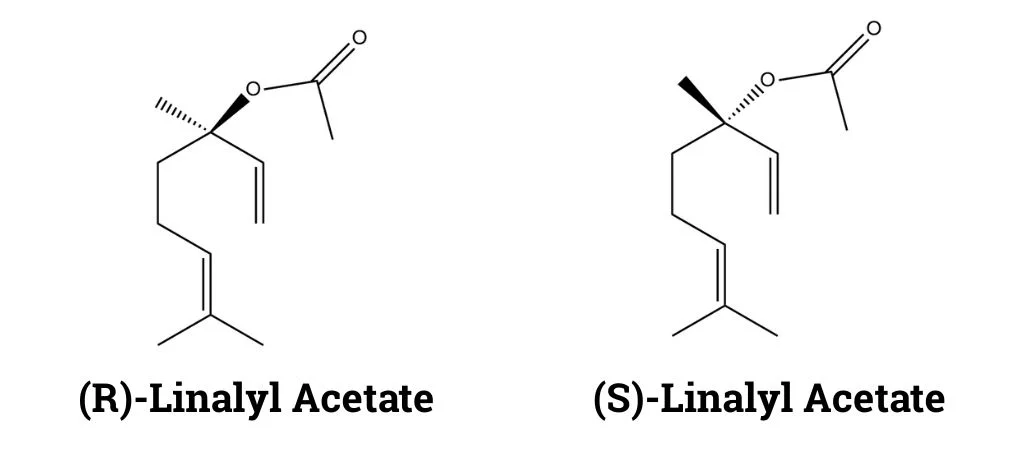
The biosynthetic pathway for linalyl acetate is almost the same as linalool, although it varies by plant species (Fowler et al., 1999). For instance, lemon mint (Mentha aquatica) obtains linalyl acetate through the acetylation of the monoterpene linalool (Zaks et al., 2008).
Like linalool, linalyl acetate also exhibits various biological activities which makes it an important commercial compound (Peana et al., 2002). Here are some of them:
- Anti-inflammatory: Linalool, and its corresponding acetate (linalyl acetate), showed potential in reducing inflammations and pain, displayed by the essential oils containing them (Peana et al., 2002).
- Analgesic & Antipyretic: Linalyl acetate may also help relieve pain and bring down fever. A 2023 study showed that linalyl acetate works by interacting with key inflammation-related pathways in the body. In higher doses, it can even outperform a standard anti-inflammatory drug in terms of how fast it worked (Alqahtani et al., 2023).
- Cardiovascular Effects: A study by Kim et al. (2017) showed that linalyl acetate treatment helped reverse cell damage and cardiovascular changes caused by nicotine exposure. This study showed that linalyl acetate has some potential as an available mitigating agent into the effects of environmental tobacco smoke.
β-Caryophyllene
β-Caryophyllene, or BCP, is a naturally occurring bicyclic sesquiterpene notable for its presence in various essential oils, including lavender (Lavandula angustifolia). In lavender, it contributes to the plant’s distinctive aroma and therapeutic properties. It’s usually found as a mixture with isocaryophyllene (the cis double bond isomer), and α-humulene, a ring-opened isomer.
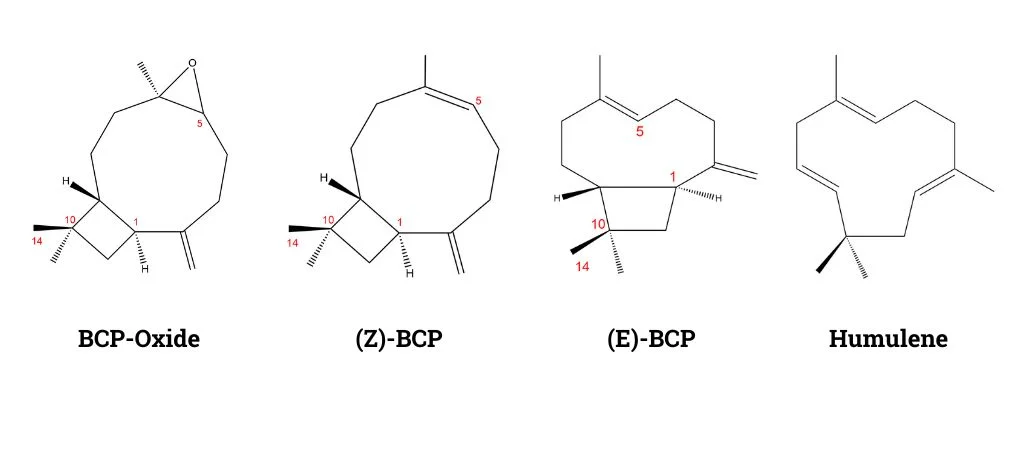
The biosynthesis of all sesquiterpenes relies on the same precursor of farnesyl diphosphate (FPP), which can be produced from the mevalonate (MVA) pathway or the MEP pathway (Cheng et al., 2022).
The MVA pathway generates IPP and DMAPP from acetyl-CoA, both of which are common substrates for terpene biosynthesis. The geranyl diphosphate synthase catalyzes IPP and DMAPP to geranyl diphosphate (GPP) and then GPP is catalyzed to FPP by farnesyl diphosphate synthase.
The FPP can then be converted to sesquiterpenes by sesquiterpene synthase.
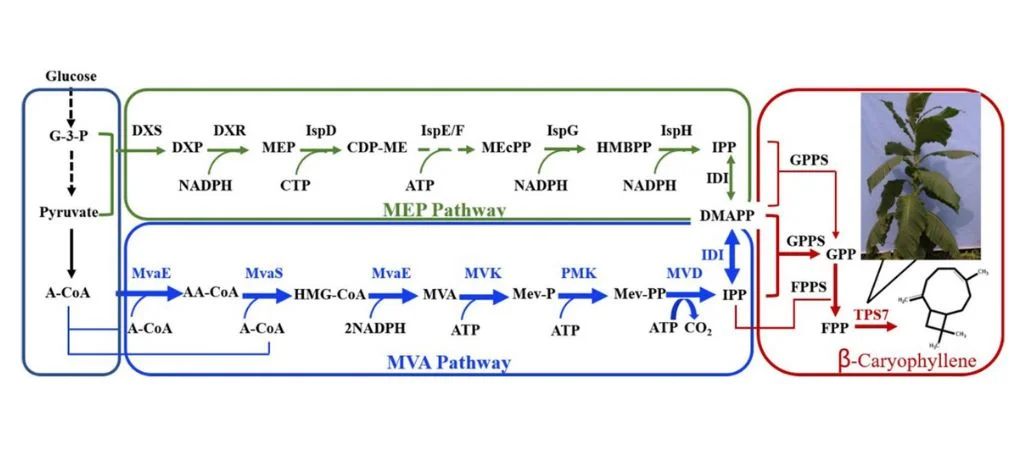
Advancements in metabolic engineering have enabled the production of BCP in microbial hosts like Escherichia coli and Yarrowia lipolytica, offering sustainable alternatives to plant extraction (Park et al., 2025).
Like linalool and linalyl acetate, BCP also showed some interesting biological properties such as a modulator of the nervous system and exerts beneficial effects on numerous neurodegenerative and inflammatory pathologies.
- Anticancer & Analgesic Properties: BCP showed promising anticancer effects by slowing down the growth of various cancer cells. Researchers found that BCP works by activating a specific receptor in the body called CB2. A related compound, β-Caryophyllene Oxide (BCPO), targets other cancer-related pathways. Together, BCP and BCPO may one day help cancer patients both feel better and respond more effectively to treatments (Fidyt et al., 2016).
- Anti-inflammatory: BCP also helped reduce inflammation in the body. A 2019 study by Francomano et al. showed that it blocks major inflammation triggers like inducible nitric oxide synthase (iNOS), Interleukin 1 beta (IL-1β), Interleukin-6 (IL-6), tumor necrosis factor alfa (TNF-α), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), cyclooxygenase 1 (COX-1), and cyclooxygenase 2 (COX-2).
- Other Biological Properties: Pre-clinical studies also demonstrated BCP’s effectiveness in fighting bacterial infections, protecting against bone loss and fatty liver diseases, and providing relief from seizures, pain, muscle tension, and even depression.
Tannins and Other Minor Compounds
The three compounds above (linalool, linalyl acetate, and BCP), comprise the major phytochemicals present in lavender. Of course, there are many more others which are equally important in making lavender a symbol of calmness, relaxation, and healing.
Tannins
Tannins are a class of astringent, polyphenolic biomolecules that bind to and precipitate proteins, including amino acids and alkaloids. They play a crucial role in protecting plants from predators (acting as pesticides) and can also help regulate plant growth.
One of the key things tannins do is bind to proteins, including those in the saliva and digestive systems of animals (including us). Tannins denature proteins when they bind to them, making them less usable or difficult to digest. But that same protein-binding ability makes tannins effective in(De Melo et al., 2023):
- Reducing harmful reactive oxygen species (ROS)
- Preventing DNA damage
- Fighting viruses and bacteria
Tannins can sometimes interfere with digestion by binding to food proteins or blocking natural enzymes in the body, which is why they’re considered anti-nutritional in some cases (Chung et al., 1998). However, this same ability to interact with proteins also helps tannins fight harmful microbes — by blocking key enzymes, damaging cell membranes, disrupting energy production, and tying up metal ions that bacteria need to grow (Guil-Guerrero et al., 2016).
Flavonoids
Some species of lavender also contain flavonoids including luteolin, apigenin, and their glycosides (Tăbărașu et al., 2023). Flavonoids in lavender, like in any other plant, are synthesized through the phenylpropanoid pathway, and their activities depend on their structures. Consequently, their chemical nature also depends on their degree of hydroxylation, polymerization, and their substitutions and conjugations.
Flavonoids are known for their antimicrobial effects on plant pests, and they also have UV protection effects against reactive oxygen species. Plant flavonoids can also slow down the aging process by protecting the body from free radical oxidation (Russo, 2018). In addition, most flavonoids have antibacterial, anticancer, anti-inflammatory, antiviral, and antifungal properties among many others (Russo, 2018).
Coumarins
Lavender plants also contain coumarins which may include umbelliferone and herniarin (Brown, 1962). Coumarins are a class of aromatic organic chemical compound with formula C9H6O2. Think of a benzene ring attached to an unsaturated lactone ring, forming a second six-membered heterocycle that shares two carbons with the benzene ring. It belongs to the benzopyrone chemical class and is considered a lactone.
The biosynthesis of coumarins in plants usually starts with the phenylpropanoid pathway, utilizing phenylalanine or tyrosine (Zou et al., 2023). These amino acids are converted into cinnamic acid and p-coumaric acid respectively by enzymes like PAL and TAL. Subsequent reactions, including hydroxylation and lactonization, lead to the formation of various coumarins.
Coumarins are one of the common organic molecules used in medicine for their pharmacological and biological effects. This includes anti-inflammatory, anticoagulant, antihypertensive, anticonvulsant, antioxidant, antimicrobial, and neuroprotective activities. In addition, coumarin derivatives can modulate signaling pathways that impact several cell processes (Flores-Morales et al., 2023).
Conclusion
From ancient rituals to essential oils and pop culture references, lavender long carried the weight of calmness, emotions, ritual, and memory. Beyond the symbolism it holds over different cultures, there’s a complex interplay of phytochemicals that make it a plant of real physiological impact.
Compounds like linalool and linalyl acetate interact with our nervous system and influences our mood, while BCP and others provide more physiological benefits. So, the next time you take a whiff of lavender, or if your playlist happens to stumble upon Taylor Swift’s song, remember, there’s chemistry behind the calm you’re feeling.
References
- Alqahtani, A., Abdelhameed, M. F., Abdou, R., Ibrahim, A. M., Dawoud, M., Alasmari, S. M., el Raey, M. A., & Attia, H. G. (2023). Mechanistic action of linalyl acetate: Acyclic monoterpene isolated from bitter orange leaf as anti-inflammatory, analgesic, antipyretic agent: Role of TNF-α, IL1β, PGE2, and COX-2. Industrial Crops and Products, 203, 117131. https://doi.org/10.1016/J.INDCROP.2023.117131
- Aprotosoaie, A. C., Hăncianu, M., Costache, I., & Miron, A. (2014). Linalool: a review on a key odorant molecule with valuable biological properties. Flavour and Fragrance Journal, 29(4), 193–219. https://doi.org/10.1002/ffj.3197
- Bradley, B., Starkey, N., Brown, S., & Lea, R. (2006). Anxiolytic effects of Lavandula angustifolia odour on the Mongolian gerbil elevated plus maze. Journal of Ethnopharmacology, 111(3), 517–525. https://doi.org/10.1016/j.jep.2006.12.021
- Brown, S. A. (1962). Biosynthesis of coumarin and herniarin in lavender. Science (New York, N.Y.), 137(3534), 977–978. https://doi.org/10.1126/SCIENCE.137.3534.977
- Buchbauer, G., Jirovetz, L., Jäger, W., Plank, C., & Dietrich, H. (1993). Fragrance Compounds and Essential Oils with Sedative Effects upon Inhalation. Journal of Pharmaceutical Sciences, 82(6), 660–664. https://doi.org/10.1002/jps.2600820623
- Cheng, T., Zhang, K., Guo, J., Yang, Q., Li, Y., Xian, M., & Zhang, R. (2022). Highly efficient biosynthesis of β-caryophyllene with a new sesquiterpene synthase from tobacco. Biotechnology for Biofuels and Bioproducts, 15(1), 1–14. https://doi.org/10.1186/S13068-022-02136-8/TABLES/1
- Chung, K. T., Wong, T. Y., Wei, C. I., Huang, Y. W., & Lin, Y. (1998). Tannins and Human Health: A Review. Critical Reviews in Food Science and Nutrition, 38(6), 421–464. https://doi.org/10.1080/10408699891274273
- Fidyt, K., Fiedorowicz, A., Strządała, L., & Szumny, A. (2016a). β‐caryophyllene and β‐caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Medicine, 5(10), 3007. https://doi.org/10.1002/CAM4.816
- Flores-Morales, V., Villasana-Ruíz, A. P., Garza-Veloz, I., González-Delgado, S., & Martinez-Fierro, M. L. (2023). Therapeutic Effects of Coumarins with Different Substitution Patterns. Molecules, 28(5), 2413. https://doi.org/10.3390/MOLECULES28052413
- Fowler, D., Hamilton, J., Humphrey, A., & O’Hagan, D. (1999). Plant terpene biosynthesis. The biosynthesis of linalyl acetate in Mentha Citrata. Tetrahedron Letters, 40, 3803–3806. https://research-portal.st-andrews.ac.uk/en/publications/plant-terpene-biosynthesis-the-biosynthesis-of-linalyl-acetate-in
- Francomano, F., Caruso, A., Barbarossa, A., Fazio, A., Torre, C. la, Ceramella, J., Mallamaci, R., Saturnino, C., Iacopetta, D., & Sinicropi, M. S. (2019). β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Applied Sciences 2019, Vol. 9, Page 5420, 9(24), 5420. https://doi.org/10.3390/APP9245420
- Guil-Guerrero, J. L., Ramos, L., Moreno, C., Zúñiga-Paredes, J. C., Carlosama-Yepez, M., & Ruales, P. (2016). Antimicrobial activity of plant-food by-products: A review focusing on the tropics. Livestock Science, 189, 32–49. https://doi.org/10.1016/J.LIVSCI.2016.04.021
- Kamatou, G. P. P., & Viljoen, A. M. (n.d.). Linalool-A Review of a Biologically Active Compound of Commercial Importance.
- Kim, J. R., Kang, P., Lee, H. S., Kim, K. Y., & Seol, G. H. (2017). Cardiovascular effects of linalyl acetate in acute nicotine exposure. Environmental Health and Preventive Medicine, 22(1), 1–7. https://doi.org/10.1186/S12199-017-0651-6/FIGURES/4
- Lavender Uses, Benefits & Dosage. (n.d.). Drugs.com. https://www.drugs.com/npp/lavender.html
- Mączka, W., Duda-Madej, A., Grabarczyk, M., & Wińska, K. (2022). Natural Compounds in the Battle against Microorganisms—Linalool. Molecules 2022, Vol. 27, Page 6928, 27(20), 6928. https://doi.org/10.3390/MOLECULES27206928
- Melo, L. F. M. de, Aquino-Martins, V. G. de Q., Silva, A. P. da, Oliveira Rocha, H. A., & Scortecci, K. C. (2023). Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chemistry, 414, 135645. https://doi.org/10.1016/J.FOODCHEM.2023.135645
- National Institute of Child Health and Human Development. (2024, November 15). Lavender. Drugs and Lactation Database (LactMed®) – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK501865/
- Park, Y. K., Studena, L., Hapeta, P., Haddouche, R., Bell, D. J., Torres-Montero, P., Martinez, J. L., Nicaud, J. M., Botes, A., & Ledesma-Amaro, R. (2025). Efficient biosynthesis of β-caryophyllene by engineered Yarrowia lipolytica. Microbial Cell Factories, 24(1), 38. https://doi.org/10.1186/S12934-025-02660-W
- Peana, A. T., D’Aquila, P. S., Panin, F., Serra, G., Pippia, P., & Moretti, M. D. L. (2002). Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine, 9(8), 721–726. https://doi.org/10.1078/094471102321621322
- Russo, D. (2018). Flavonoids and the Structure-Antioxidant Activity Relationship. https://doi.org/10.4172/2472-0992.1000e109
- Shaw, D., Annett, J., Doherty, B., & Leslie, J. (2007). Anxiolytic effects of lavender oil inhalation on open-field behaviour in rats. Phytomedicine, 14(9), 613–620. https://doi.org/10.1016/j.phymed.2007.03.007
- Tăbărașu, A. M., Anghelache, D. N., Găgeanu, I., Biriș, S. Ștefan, & Vlăduț, N. V. (2023). Considerations on the Use of Active Compounds Obtained from Lavender. Sustainability 2023, Vol. 15, Page 8879, 15(11), 8879. https://doi.org/10.3390/SU15118879
- Umezu, T., Nagano, K., Ito, H., Kosakai, K., Sakaniwa, M., & Morita, M. (2006). Anticonflict effects of lavender oil and identification of its active constituents. Pharmacology Biochemistry and Behavior, 85(4), 713–721. https://doi.org/10.1016/j.pbb.2006.10.026
- Wang, K., Ren, W., Hong, L., Wang, Q., Ghimire, R., Haapanen, M., Kivimäenpää, M., Wu, P., Ma, X., & Asiegbu, F. O. (2024). Linalool and 1,8-Cineole as Constitutive Disease-Resistant Factors of Norway Spruce Against Necrotrophic Pathogen Heterobasidion Parviporum. Plant Cell and Environment. https://doi.org/10.1111/pce.15280
- Wolkoff, P., & Nielsen, G. D. (2017). Effects by inhalation of abundant fragrances in indoor air – An overview. Environment International, 101, 96–107. https://doi.org/10.1016/J.ENVINT.2017.01.013
- Zaks, A., Rachel, D. R., Bar, E., Inbar, M., & Lewinsohn, E. (2008). Biosynthesis of linalyl acetate and other terpenes in lemon mint (Mentha aquatica var. citrata, Lamiaceae) glandular trichomes. Israel Journal of Plant Sciences, 56(3), 233–244. https://doi.org/10.1560/IJPS.56.3.233
- Zou, Y., Teng, Y., Li, J., & Yan, Y. (2024). Recent advances in the biosynthesis of coumarin and its derivatives. Green Chemical Engineering, 5(2), 150–154. https://doi.org/10.1016/J.GCE.2023.04.003

